Dr. Belmonte Recent Publications
- A computational dynamic systems model for in silico prediction of neural tube closure defects. JH Berkhout, JA Glazier, AH Piersma, JM Belmonte, J Legler, RM Spencer, TB Knudsen, HJ Heusinkveld. Current Research in Toxicology (in press), 2024.
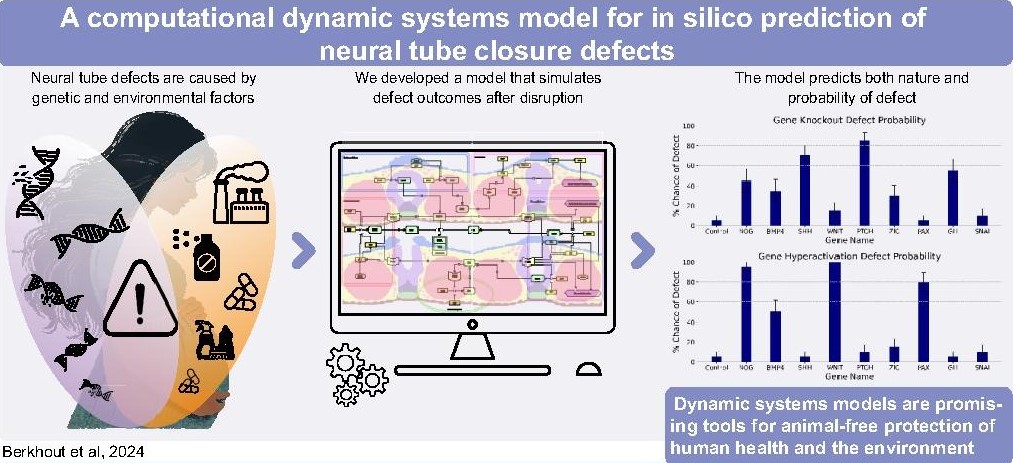
Abstract: Neural tube closure is a critical morphogenetic event during early vertebrate development. This complex process is susceptible to perturbation by genetic errors and chemical disruption, which can induce severe neural tube defects (NTDs) such as spina bifida. We built a computational agent-based model (ABM) of neural tube development based on the known biology of morphogenetic signals and cellular biomechanics underlying neural fold elevation, bending and fusion. The computer model functionalizes cell signals and responses to render a dynamic representation of neural tube closure. Perturbations in the control network can then be introduced synthetically or from biological data to yield quantitative simulation and probabilistic prediction of NTDs by incidence and degree of defect. Translational applications of the model include mechanistic understanding of how singular or combinatorial alterations in gene-environmental interactions and animal-free assessment of developmental toxicity for an important human birth defect (spina bifida) and potentially other neurological problems linked to development of the brain and spinal cord.
- Caging of membrane-to-cortex attachment proteins can trigger cellular symmetry breaking. Srishti Dar, Ruben Tesoro Moreno, Ivan Palaia, Anusha Bargavi Gopalan, Zachary Gao Sun, Leanne Strauss, Richard Springer, Julio Monti Belmonte, Sarah Katherine Foster, Michael Murrell, Christer Stenby Ejsing, Andela Saric, Maria Leptin, Alba Diz-Munoz. bioRxiv, 2024.
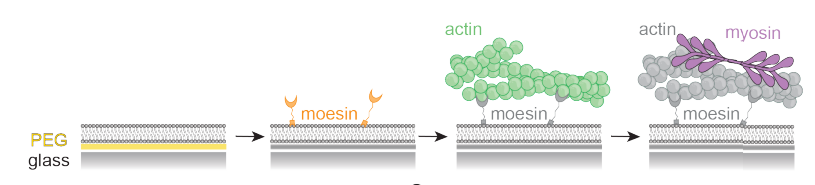
Abstract: To migrate, divide, and change shape, cells must regulate the mechanics of their periphery. The cell surface is a complex structure that consists of a thin, contractile cortical actin network tethered to the plasma membrane by specialized membrane-to-cortex attachment (MCA) proteins. This active and constantly fluctuating system maintains a delicate mechanochemical state which permits spontaneous polarization and shape change when needed. Combining in silico, in vitro, and in vivo experiments we show how membrane viscosity and MCA protein length regulate cortical dynamics. We reveal a novel mechanism whereby caging of linker proteins in the actin cortex allows for the amplification of small changes in these key parameters, leading to major alterations of cortical contractility. In cells, this mechanism alone gives rise to symmetry breaking phenomena, suggesting that local changes in lipid composition, in combination with the choice of MCA proteins, contribute to the regulation of cellular morphogenesis and function.
- Highly dynamic mechanical transitions in embryonic cell populations during Drosophila gastrulation. Juan Manuel Gomez, Carlo Bevilacqua, Abhisha Thayambath, Maria Leptin, Julio Belmonte, Robert Prevedel. bioRxiv, 2024.
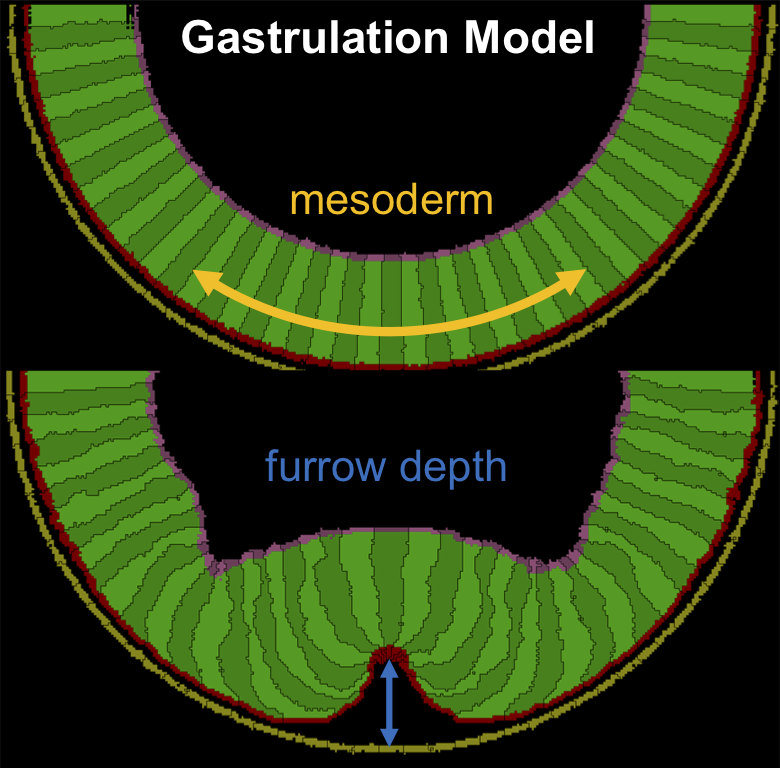
Abstract:During animal development, the acquisition of three-dimensional morphology is a direct consequence of the dynamic interaction between cellular forces and the mechanical properties of cells and their environment. While the generation and transmission of cellular forces has been widely explored, less is known about the dynamic changes in cell mechanical properties during morphogenesis. Here, we characterise and spatially map in three dimensions the dynamics of cell mechanical properties during Drosophila gastrulation utilising line-scan Brillouin microscopy. We find that cells in the embryo undergo rapid and spatially varying changes in their mechanical properties and that these differ in cell populations with different fates and behaviours. We identify microtubules as potential effectors of cell mechanics in this system, and corroborate our experimental findings with a physical model that underscores the role of localised and dynamic changes in mechanical properties to facilitate tissue folding. Our work provides the first spatio-temporal description of the evolving mechanical properties of cell populations during morphogenesis, and highlights the potential of Brillouin microscopy in studying the dynamic changes in cell shape behaviours and cell mechanical properties simultaneously in different cell populations in an intact organism.
- Microtubule competition and cell growth recenter the nucleus after anaphase in fission yeast. K Bellingham-Johnstun, A Thorn, JM Belmonte, C Laplante. Molecular Biology of the Cell 34(8): E23-01-0034, 2023.
Abstract:  Cells actively position their nucleus based on their activity. In fission yeast, microtubule-dependent nuclear centering is critical for symmetrical cell division. After spindle disassembly at the end of anaphase, the nucleus recenters over a ~90 min period, approximately half of the duration of the cell cycle. Live cell and simulation experiments support the cooperation of two distinct mechanisms in the slow recentering of the nucleus. First, a push-push mechanism acts from spindle disassembly to septation and involves the opposing actions of the mitotic Spindle Pole Body microtubules that push the nucleus away from the ends of the cell while post-anaphase array of microtubules basket the nucleus and limit its migration toward the division plane. Second, a slow-and-grow mechanism finalizes nuclear centering in the newborn cell. In this mechanism, microtubule competition stalls the nucleus while asymmetric cell growth slowly centers it. Our work underlines how intrinsic properties of microtubules differently impact nuclear positioning according to microtubule network organization and cell size.
Cells actively position their nucleus based on their activity. In fission yeast, microtubule-dependent nuclear centering is critical for symmetrical cell division. After spindle disassembly at the end of anaphase, the nucleus recenters over a ~90 min period, approximately half of the duration of the cell cycle. Live cell and simulation experiments support the cooperation of two distinct mechanisms in the slow recentering of the nucleus. First, a push-push mechanism acts from spindle disassembly to septation and involves the opposing actions of the mitotic Spindle Pole Body microtubules that push the nucleus away from the ends of the cell while post-anaphase array of microtubules basket the nucleus and limit its migration toward the division plane. Second, a slow-and-grow mechanism finalizes nuclear centering in the newborn cell. In this mechanism, microtubule competition stalls the nucleus while asymmetric cell growth slowly centers it. Our work underlines how intrinsic properties of microtubules differently impact nuclear positioning according to microtubule network organization and cell size.
- β-heavy-spectrin stabilizes the constricting contractile ring during cytokinesis. Ana Marta Silva, Fung-Yi Chan, Michael J Norman, Ana Filipa Sobral, Esther Zanin, Reto Gassmann, Julio Monti Belmonte, Ana Xavier Carvalho. Journal of Cell Biology 222 (1), e202202024, 2022.
Abstract:  Cytokinesis requires the constriction of an actomyosin-based contractile ring and involves multiple F-actin crosslinkers. We show that partial depletion of the C. elegans cytokinetic formin generates contractile rings with low F-actin levels that constrict but are structurally fragile, and we use this background to investigate the roles of the crosslinkers plastin/PLST-1 and β-heavy-spectrin/SMA-1 during ring constriction. We show that the removal of PLST-1 or SMA-1 has opposite effects on the structural integrity of fragile rings. PLST-1 loss reduces cortical tension that resists ring constriction and makes fragile rings less prone to ruptures and regressions, whereas SMA-1 loss exacerbates structural defects, leading to frequent ruptures and cytokinesis failure. Fragile rings without SMA-1 or containing a shorter SMA-1, repeatedly rupture at the same site, and SMA-1::GFP accumulates at repair sites in fragile rings and in rings cut by laser microsurgery. These results establish that β-heavy-spectrin stabilizes the constricting ring and reveals the importance of β-heavy-spectrin size for network connectivity at low F-actin density.
Cytokinesis requires the constriction of an actomyosin-based contractile ring and involves multiple F-actin crosslinkers. We show that partial depletion of the C. elegans cytokinetic formin generates contractile rings with low F-actin levels that constrict but are structurally fragile, and we use this background to investigate the roles of the crosslinkers plastin/PLST-1 and β-heavy-spectrin/SMA-1 during ring constriction. We show that the removal of PLST-1 or SMA-1 has opposite effects on the structural integrity of fragile rings. PLST-1 loss reduces cortical tension that resists ring constriction and makes fragile rings less prone to ruptures and regressions, whereas SMA-1 loss exacerbates structural defects, leading to frequent ruptures and cytokinesis failure. Fragile rings without SMA-1 or containing a shorter SMA-1, repeatedly rupture at the same site, and SMA-1::GFP accumulates at repair sites in fragile rings and in rings cut by laser microsurgery. These results establish that β-heavy-spectrin stabilizes the constricting ring and reveals the importance of β-heavy-spectrin size for network connectivity at low F-actin density.
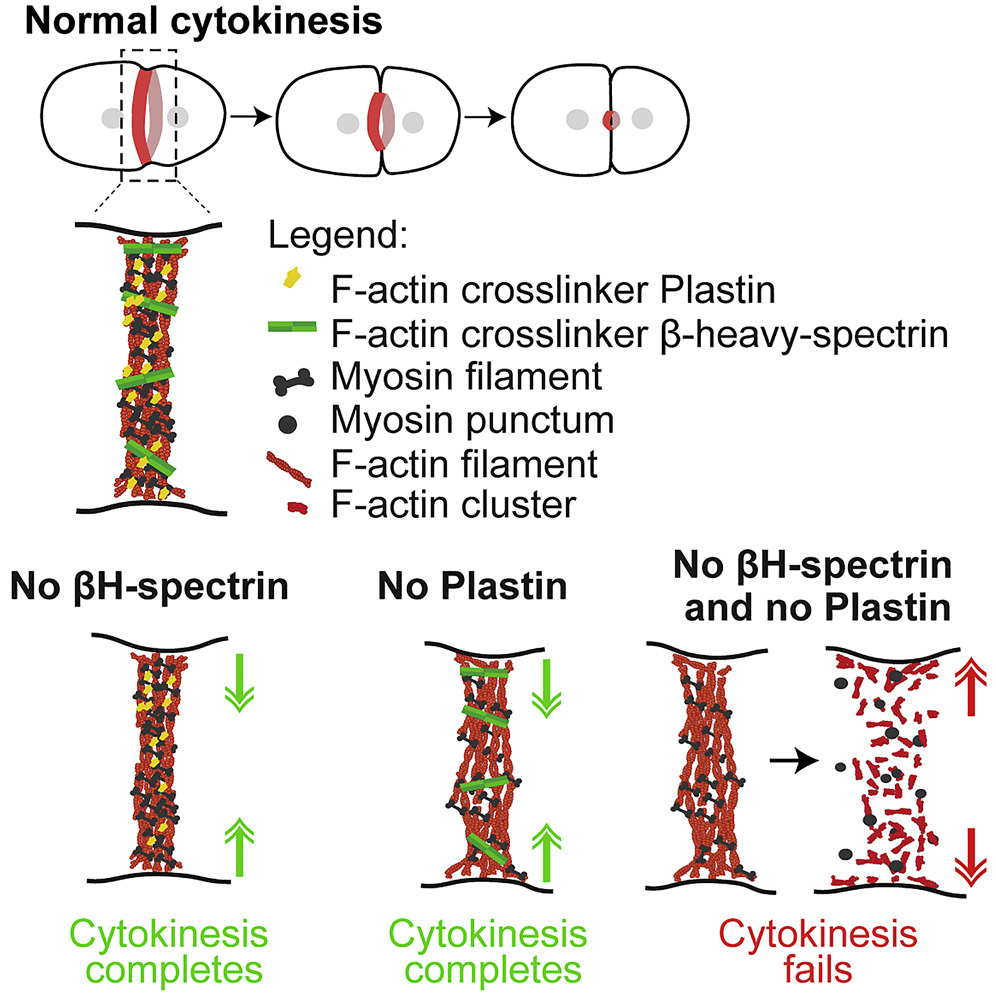
- Plastin and spectrin cooperate to stabilize the actomyosin cortex during cytokinesis. Ana Filipa Sobral, Fung-Yi Chan, Michael J Norman, Daniel S Osório, Ana Beatriz Dias, Vanessa Ferreira, Daniel J Barbosa, Dhanya Cheerambathur, Reto Gassmann, Julio Monti Belmonte, Ana Xavier Carvalho. Current Biology 31 (24), 5415-5428. e10, 2021.
Abstract: Cytokinesis, the process that partitions the mother cell into two daughter cells, requires the assembly and constriction of an equatorial actomyosin network. Different types of non-motor F-actin crosslinkers localize to the network, but their functional contribution remains poorly understood. Here, we describe a synergy between the small rigid crosslinker plastin and the large flexible crosslinker spectrin in the C. elegans one-cell embryo. In contrast to single inhibitions, co-inhibition of plastin and the βH-spectrin (SMA-1) results in cytokinesis failure due to progressive disorganization and eventual collapse of the equatorial actomyosin network. Cortical localization dynamics of non-muscle myosin II in co-inhibited embryos mimic those observed after drug-induced F-actin depolymerization, suggesting that the combined action of plastin and spectrin stabilizes F-actin in the contractile ring. An in silico model predicts that spectrin is more efficient than plastin at stabilizing the ring and that ring formation is relatively insensitive to βH-spectrin length, which is confirmed in vivo with a sma-1 mutant that lacks 11 of its 29 spectrin repeats. Our findings provide the first evidence that spectrin contributes to cytokinesis and highlight the importance of crosslinker interplay for actomyosin network integrity.